
Also, all fluorine atoms bonded to carbon atoms lie at the corners of the tetrahedron with a 109.5º bond angle between them. So, the molecular geometry of CF4 is tetrahedral. In the case of CF4, all fluorine atoms attached to a carbon atom, and no lone pair present on the central atom. A) 0 lone pairs, square planar D) 1 lone pair, trigonal bipyramidal B) 0 lone pairs, tetahedral E) 2 lone pairs, square planar With a net dipole moment of zero, the molecule is nonpolar.
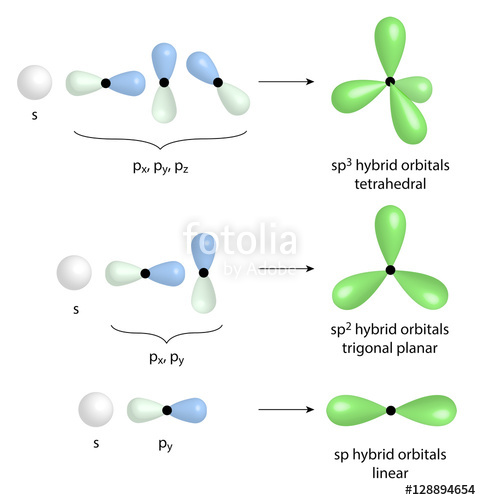
Tetrahedral- CF4 Trigonal Pyramidal- NF3 Bent- OF2 and H2S. Tetrahedral What is the electron geometry of cs2?ĬS2 – Carbon Disulfide: The molecular geometry of CS2 is linear with symmetric electron region distribution around the central atom. Hybridization of XeO2F2 (Xenon Dioxide Difluoride) Name of the Molecule What is the hybridization and geometry of XeOF2?

Is nitrogen and oxygen a covalent bond?.



 0 kommentar(er)
0 kommentar(er)
